FDA Modernization Act 2.0 Accelerates Shift to Non-Animal Drug Testing Methods
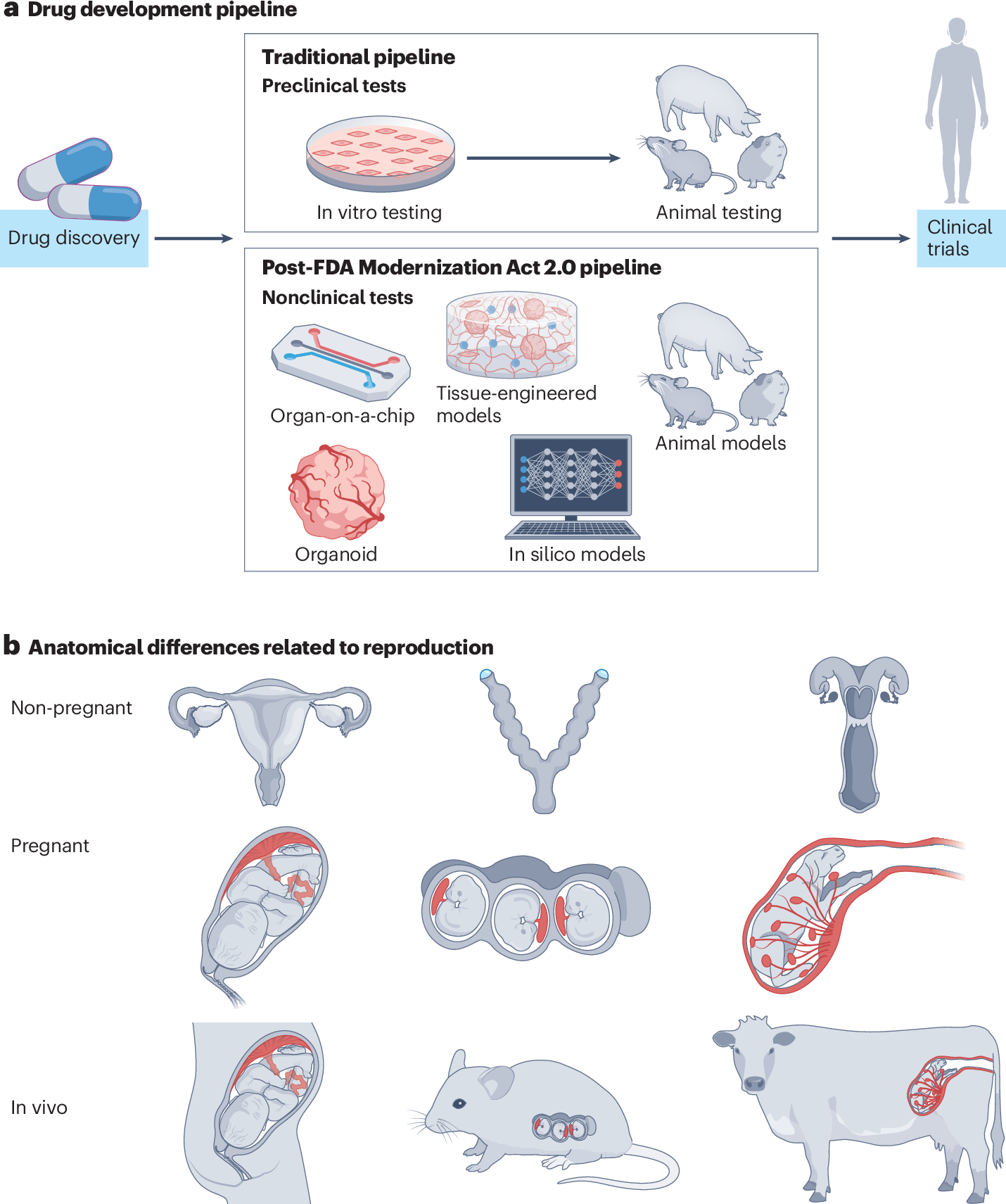
A significant shift in pharmaceutical research and development is underway, driven by ethical considerations, scientific advancements, and regulatory changes, particularly the FDA Modernization Act 2.0. This landmark legislation, signed into law in December 2022, has removed the long-standing federal mandate for animal testing in new drug applications, paving the way for alternative testing methods. The move is expected to enhance drug discovery efficiency and accuracy, ultimately saving human lives.
Parmita Mishra, CEO of Precigenetics, highlighted the limitations of traditional animal models, stating, "The greatest biologists in the world are too often constrained to rodents. And I believe that this is costing human lives. And it’s time to build better in vitro." Mishra, whose company focuses on using biophotonics, AI, and single-cell epigenomics to combat stem cell-like cancers, advocates for advanced human-relevant models. The traditional reliance on animal models has resulted in a high failure rate in clinical trials, with over 90% of drugs successful in animal studies failing in human trials due to fundamental species differences.
The FDA Modernization Act 2.0 explicitly permits the use of New Approach Methodologies (NAMs), including cell-based assays, organ-on-a-chip systems, and advanced computational models like AI and machine learning, to establish drug safety and efficacy. This regulatory change acknowledges the scientific limitations of animal models, which often fail to predict human responses due to genetic homogeneity in lab animals compared to human genetic diversity. The FDA's "Roadmap to reducing animal testing in preclinical safety studies," published in April 2025, outlines a stepwise approach to integrate these validated NAMs.
In vitro human-based systems, such as organoids—miniature, lab-grown versions of human organs—and organ-on-a-chip devices, offer more physiologically relevant insights into disease mechanisms and drug responses. These models allow researchers to study human organ-level biology in a dish, potentially detecting tissue-specific responses and toxicities that animal models miss. Concurrently, in silico approaches leverage computational modeling and AI to predict drug behavior, toxicity, and pharmacokinetics, accelerating drug design and optimizing clinical trial strategies. Companies like Roche, Johnson & Johnson, AstraZeneca, and Insilico Medicine are actively investing in and partnering to integrate these technologies.
The transition, while facing challenges in scientific validation and integration, promises faster, more cost-effective, and accurate predictions of human-relevant outcomes. This paradigm shift aims to reduce the time and cost of drug development, leading to more reliable and successful therapies reaching patients. The FDA's proactive stance and industry's increasing adoption of NAMs signal a new era in drug discovery, moving towards a future where human-centric testing methods become the standard.