First Personalized CRISPR Therapy Developed in Six Months Transforms Infant's Life with Rare Genetic Disease
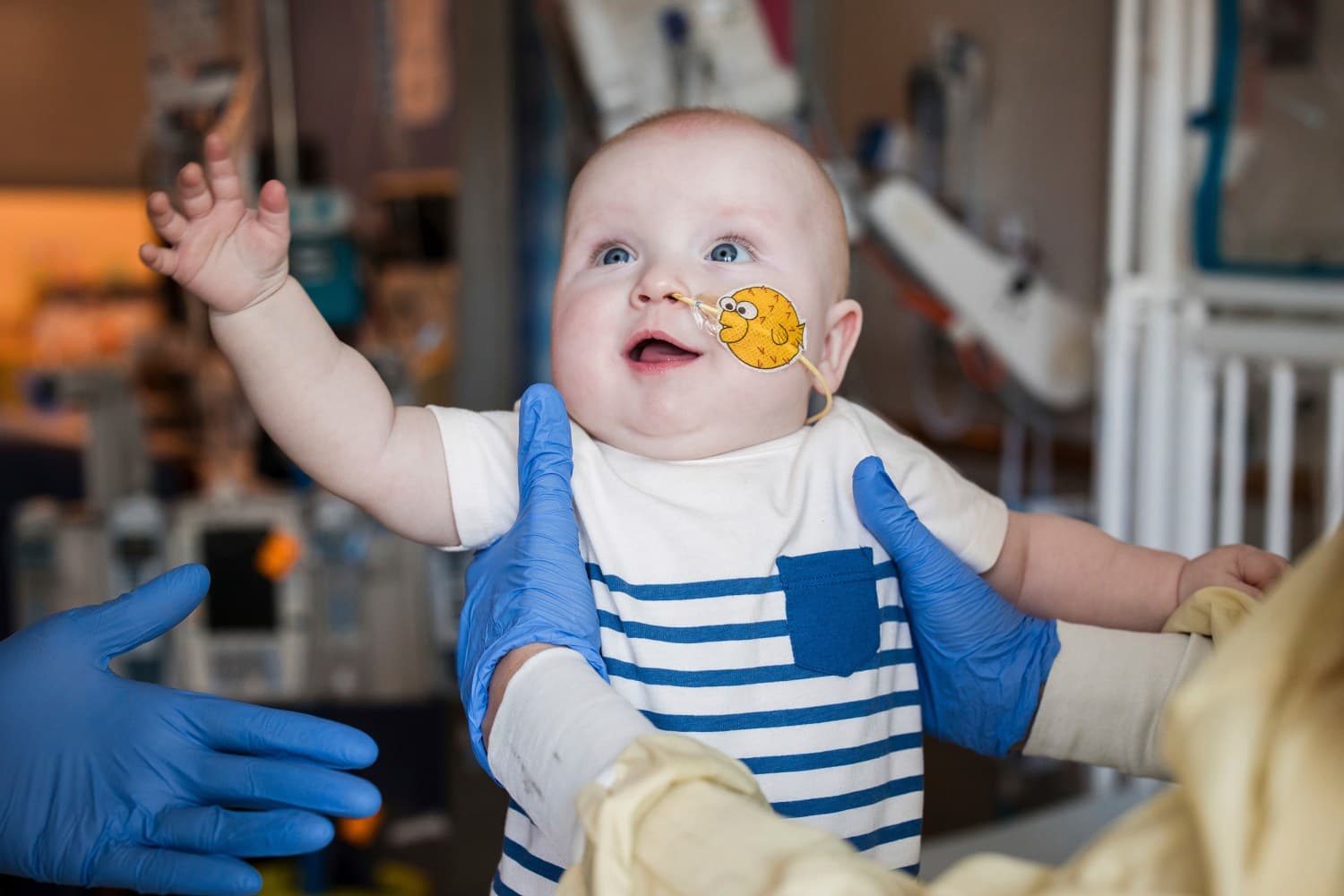
Philadelphia, PA – A groundbreaking "N of 1" gene therapy, custom-designed and delivered in just six months, has dramatically improved the life of an infant diagnosed with a severe and ultra-rare genetic disorder. The case, detailed in the New England Journal of Medicine and presented at the American Society of Gene & Cell Therapy meeting, marks a significant milestone in personalized medicine.
The infant, identified as KJ Muldoon, was born with severe carbamoyl phosphate synthetase 1 (CPS1) deficiency, a condition affecting approximately one in 1.3 million newborns. This disorder prevents the liver from properly processing ammonia, leading to toxic buildup that can cause severe brain damage or death. Traditional treatment often involves a restrictive diet and medication, with a liver transplant as a later option, but many infants do not survive long enough for a transplant.
Researchers at the Children’s Hospital of Philadelphia (CHOP) and the University of Pennsylvania, led by Dr. Rebecca Ahrens-Nicklas and Dr. Kiran Musunuru, spearheaded the rapid development of the bespoke therapy. The treatment utilizes CRISPR base editing, a precise gene-editing technique that corrects a single "misspelling" in the baby's DNA. This genetic fix is delivered directly to liver cells via lipid nanoparticles.
The urgency of KJ's condition prompted an unprecedented collaborative effort involving academic institutions, industry partners, and regulatory bodies like the FDA, which expedited approval within a week. Eryney Marrogi, who wrote about the breakthrough for Core Memory, captured the sentiment, stating in a tweet, "Rarely do you read a paper and know instantly that it means things have changed completely. ...Come read about the N of 1 gene therapy that changed a baby’s life, and what it means for the future of genetic disease." Marrogi also acknowledged the contributions of Dr. Kiran Musunuru, Dr. David Liu (whose lab invented the base-editing method), and Dr. Petros Giannikopoulos.
Following multiple doses of the therapy, KJ has shown remarkable progress, tolerating increased protein intake and requiring significantly less nitrogen-scavenging medication. His improved health allowed him to recover from common childhood illnesses without dangerous ammonia spikes. This pioneering "N of 1" approach offers a potential blueprint for treating thousands of other ultra-rare genetic diseases, signaling a new era for personalized, life-saving medical interventions.