Deep Learning Model Achieves Over 84% Accuracy in Predicting Tumor Drug Response
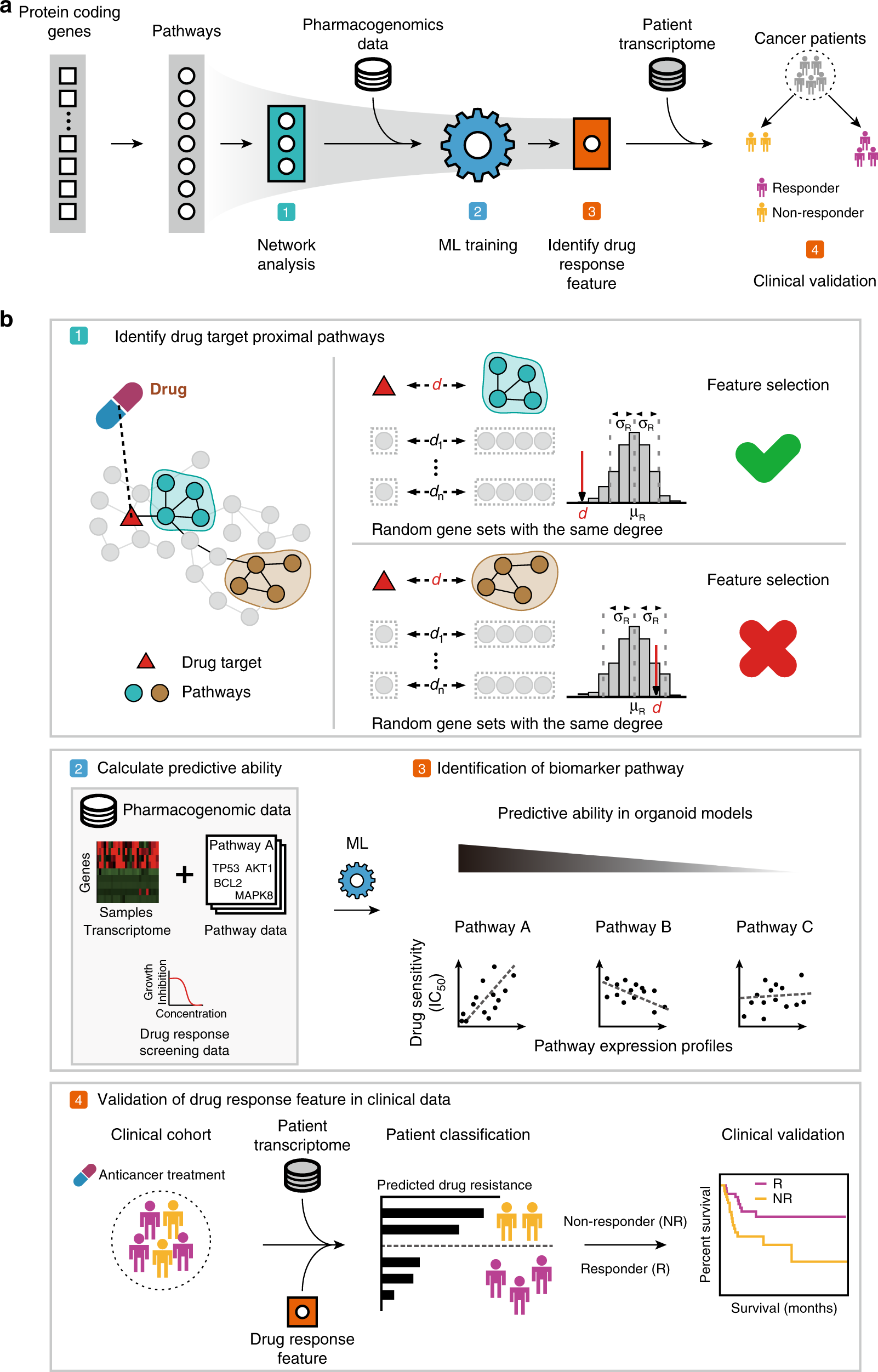
Seoul, South Korea – A novel deep learning model, Cancer Drug Response Profile scan (CDRscan), has demonstrated remarkable accuracy, achieving an R² value exceeding 0.84 and an AUROC over 0.98, in predicting how tumor cells will respond to experimental drugs. This advancement, detailed in a study published in Nature Scientific Reports, leverages "virtual cells" and "virtual drugs" to simulate drug interactions, offering a significant step forward in precision oncology and drug repurposing.
The CDRscan model, developed by researchers including Yoosup Chang, Hyejin Park, and Hyun-Jin Yang, utilizes a dual convergence architecture. This design processes genomic mutational fingerprints of 787 human cancer cell lines and structural profiles of 244 drugs individually before merging them through an "in silico virtual docking" mechanism. This innovative approach allows for the prediction of drug effectiveness based on a tumor's unique genomic signature.
According to the study, the model's high prediction accuracy was consistent across 25 different cancer types, with R² values ranging from 0.77 to 0.89. This robust performance is particularly significant given the complexity of linking mutation profiles to drug efficacy. The researchers noted that while gene expression data has often been considered the best predictor, genomic profile-based algorithms like CDRscan are increasingly in demand due to the growing prevalence of genomic sequencing in clinical practice.
A key application of CDRscan is its potential for drug repurposing. The model was applied to 1,487 approved drugs, identifying 14 oncology and 23 non-oncology drugs with new potential cancer indications. This marks a pioneering application of deep learning in systematically predicting drug repurposing feasibility, potentially reducing the time and cost associated with new drug development.
The development of CDRscan represents a crucial milestone for precision cancer medicine. By providing a tool that can accurately predict drug sensitivity from genomic data, it aims to streamline the selection of the most effective anticancer drugs for individual patients. Further clinical validation is anticipated to integrate this technology into routine decision-making processes for patient-tailored cancer treatments.