Gene Editing Extends Progeria Mouse Lifespan by Over 140% as Human Trials Loom
Recent scientific advancements, particularly in gene editing, are bringing renewed hope for a potential cure for Progeria, a rare genetic disorder that causes rapid, premature aging in children. The New Yorker recently highlighted these breakthroughs, noting that scientists "may be closing in on a cure" for the disease, which can cause children's bodies to age eight or nine decades by their teenage years. These developments follow decades of dedicated research and promising results in preclinical studies.
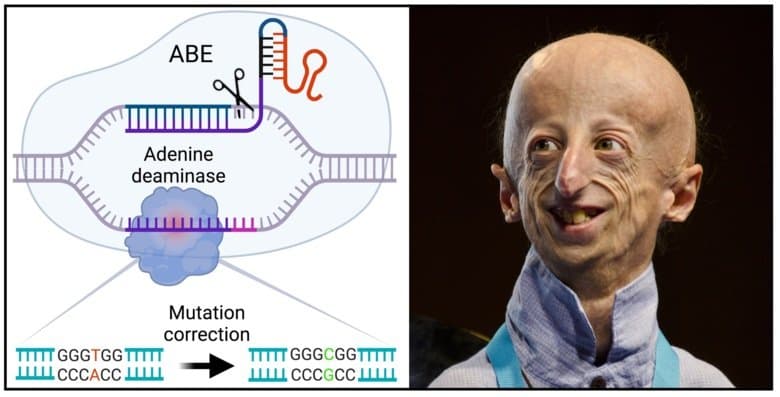
Hutchinson-Gilford Progeria Syndrome (HGPS) is caused by a single-point mutation in the LMNA gene, leading to the production of an abnormal protein called progerin. This toxic protein destabilizes the cell nucleus, resulting in severe symptoms like stunted growth, hair loss, wrinkled skin, and critical cardiovascular issues, often leading to death around 14.5 years of age. While existing treatments like Lonafarnib (Zokinvy) have shown to extend lifespan by approximately 1.6 to 2.5 years, gene editing offers a more fundamental approach by correcting the root genetic defect.
A collaborative team, including Dr. Francis Collins, former Director of the National Institutes of Health, and Dr. David Liu of Harvard University, has made significant strides using base editing technology. This precise gene editing method corrects the specific DNA mutation in the LMNA gene. In mouse models of Progeria, this base editing approach dramatically increased lifespan by an astounding 140%, with treated mice living around 510 days compared to 215 days for untreated mice.
Beyond lifespan extension, the base editing treatment in mice also showed remarkable reversal of severe symptoms, including significant improvements in vascular health and the prevention of heart damage. The Progeria Research Foundation (PRF), co-founded by Dr. Leslie Gordon, whose son had Progeria, has been instrumental in funding and coordinating these research efforts. The success in mouse models has paved the way for planning human clinical trials, with researchers aiming to initiate trials within the next two years.
In a separate but equally promising development, a team led by Dr. Sun-Uk Kim in South Korea has developed an RNA-based therapy using molecular "scissors" (RfxCas13d) to precisely target and remove the mutant progerin RNA. This approach, which modifies RNA rather than DNA, offers a potentially safer and reversible treatment option. Studies in mouse models demonstrated significant reversal of hallmark Progeria symptoms, including hair loss, skin atrophy, and improved organ function, resembling healthy controls.
The progress in gene and RNA editing for Progeria holds immense implications not only for the approximately 100-150 known children worldwide affected by HGPS but also for the broader field of rare genetic diseases and aging research. Researchers are actively seeking manufacturing partners and regulatory approval to translate these preclinical successes into effective human therapies. The scientific community remains hopeful that these cutting-edge interventions will lead to a definitive cure.