Individual Reports Dramatic Cognitive Improvement with Retatrutide, Sparks Discussion on Unexplored Benefits
A recent social media post from a prominent online personality, SwiftOnSecurity, has drawn attention to a surprising, unproven effect of the experimental drug Retatrutide. The individual claimed a significant restoration of cognitive function, stating, "I hit a dose and it's like my brain has completely switched to the functionality I had 7+ years ago." This personal account contrasts with their experience on Tirzepatide, which "didn't do this."
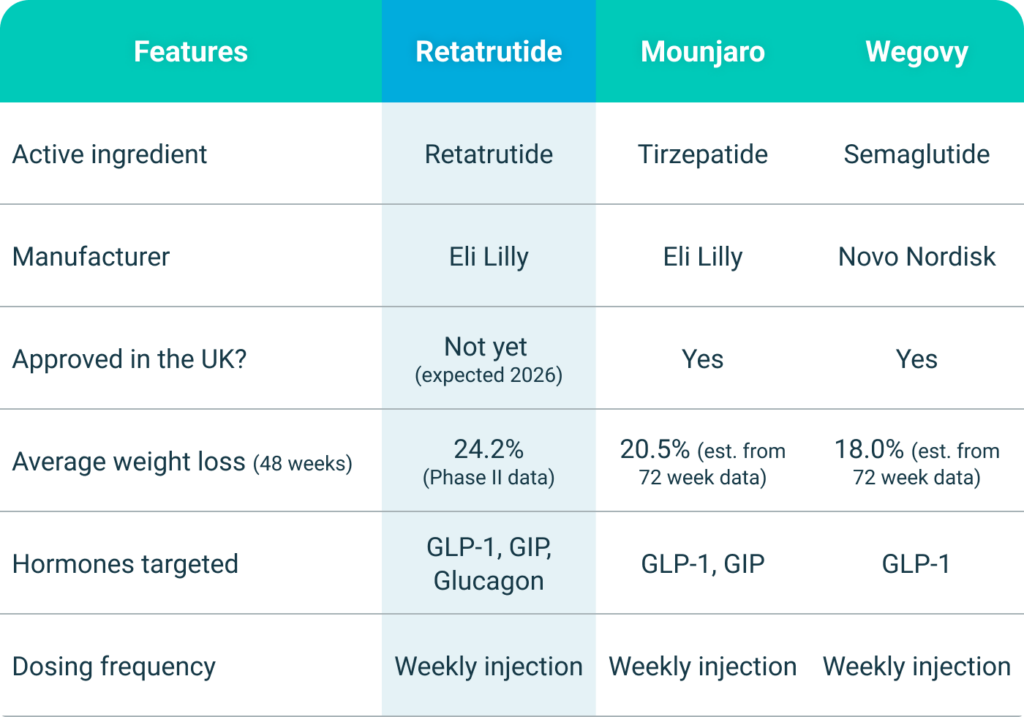
Retatrutide, developed by Eli Lilly, is a triple-hormone-receptor agonist targeting GIP, GLP-1, and glucagon receptors. It is primarily known for its substantial efficacy in weight reduction and glycemic control, as demonstrated in phase 2 clinical trials. These studies have shown dose-dependent weight loss of up to 24.2% after 48 weeks and significant improvements in HbA1c levels for individuals with obesity and type 2 diabetes.
Despite these established benefits, current scientific literature and clinical trials for Retatrutide do not report cognitive enhancement as a known effect. Research indicates that the most common adverse events are gastrointestinal, such as nausea, diarrhea, and vomiting, consistent with other GLP-1 receptor agonists. There are currently no registered trials specifically assessing Retatrutide's impact on cognition or dementia.
Similarly, Tirzepatide, a dual GIP and GLP-1 receptor agonist, is also recognized for its effectiveness in managing type 2 diabetes and obesity. While some GLP-1 receptor agonists have been explored for potential neuroprotective effects in small studies, robust evidence for direct cognitive improvement from either Tirzepatide or Retatrutide is lacking in the broader scientific community.
The tweet highlights a potential area for future research into the broader effects of these metabolic drugs, beyond their primary indications. While individual experiences can be compelling, medical professionals emphasize the importance of evidence-based research to confirm such claims and understand any underlying mechanisms.